SBC101 CASE
Study: Technological Advances and Protective Measures Against Central Venous Catheter-Related Bloodstream Infections
Highlighting the importance of prevention and care of bloodstream infections related to central venous catheters (CVCs) in Veracruz, Mexico for the entire world, promoting and educating so that people understand the importance of caring for our blood, sharing knowledge, and creating new perspectives for everyone, not just healthcare personnel.
Introduction
Nosocomial bloodstream infections are a significant cause of morbidity and mortality. They can be primary or secondary, with primary infections, most commonly associated with central venous catheters (CVCs), occurring mainly in Intensive Care Units (ICUs). Secondary infections are related to infections at other sites, such as the lungs, urinary tract, surgical wounds, etc.
The use of vascular catheters can sometimes lead to local or systemic infections, such as uncomplicated or complicated bacteremia (persistent bacteremia, septic thrombophlebitis, endocarditis, and other metastatic complications such as pulmonary abscesses, cerebral abscesses, osteomyelitis, and endophthalmitis). These complications have significant morbidity and non-negligible mortality, being the most common cause requiring removal of any type of device.
Catheter-related bloodstream infections (CRBSIs) are among the most common hospital-acquired infections. Currently, it is estimated that between 15 and 30% of all nosocomial bacteremias are related to the use of percutaneous central venous catheters (CVCs). In certain hospital units, such as ICUs, this type of infection has been associated with high morbidity, attributable mortality, and very relevant additional healthcare costs. Although the true incidence of CRBSIs is not well known, it is estimated that in the United States in 2002, a total of 250,000 episodes occurred, with an attributable mortality that can range between 12 and 25% (more than 30,000 deaths) and an estimated additional cost ranging from 3,000 to 56,167 US dollars per episode. A substantial portion of CRBSIs is associated with the presence of a CVC and patients’ stay in the ICU, although in recent years, the importance of the problem has also been documented in hospitalized patients in conventional units and with other types of catheters, such as peripheral venous catheters (PVCs) or peripherally inserted central venous catheters (PICCs), which have significant use outside of ICUs.
Surveillance programs for the prevention of CRBSIs, mainly aimed at the simple application of a group of proven preventive measures, associated with educational campaigns aimed at personnel and implemented in collaboration with the management structures of institutions, have had a significant impact on reducing CRBSI rates in ICUs. Strict adherence to the recommendations has facilitated a 70% reduction in the frequency of episodes in North American ICUs. In 2009, the estimated number of CRBSI episodes was 18,000: a 58% reduction compared to the data for the year 2001.
In Spain, since 1994, there has been a specific program for monitoring infections associated with devices acquired during patients’ stay in ICUs (called ENVIN-ICU), with more than 100 participating hospitals. The annual information provided by this program offers CRBSI rates adjusted by different methods, such as days of use of the devices, length of stay in these units, or the number of patients admitted to them. With this data, comparisons of frequencies can be made, not only in each of the participating centers but also with the aggregated data provided, allowing the implementation of specific prevention programs and evaluating the effectiveness of the measures implemented in them. However, to date, there is no precise information in our setting about the frequency of CRBSI in conventional hospital wards. In 2006, the VINCat program for the surveillance of nosocomial infections was started in Catalonia, with the main objective of reducing the frequency of these infections through active and continuous surveillance. A key objective of the VINCat program is the continuous monitoring of CRBSIs throughout the hospital and in all types of venous catheters (with the exception of CVCs with implantable reservoirs and arterial catheters), using a system based on reports of positive blood cultures from the microbiology laboratories of each participating institution.
The incidence of CRBSI varies considerably depending on the type of catheter, its frequency of manipulation, and factors related to the host (e.g., underlying disease or critical clinical condition). Although PVCs have a low risk of related infections, they can sometimes cause processes of special associated morbidity and mortality. Most serious catheter-related infections occur in patients with CVCs, especially if they are admitted to the ICU or have serious underlying conditions such as neoplasms, chronic renal failure on hemodialysis, or treatment with parenteral nutrition (PN) or immunosuppressants.
CVCs can be inserted using 2 different techniques, percutaneously or with a surgical procedure. The former is the most commonly used in hospitalized patients, and the most common central insertion sites are the subclavian, jugular, or femoral veins.
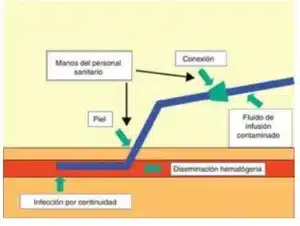
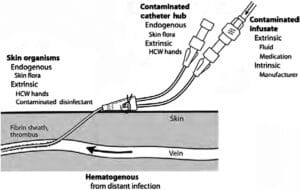
Microorganisms causing infections related to intravascular devices can access them through either an extraluminal or an intraluminal route. The adherence of these microorganisms and their incorporation into biofilms leads to catheter colonization, with the possibility of hematogenous dissemination.
There are 3 important points where microorganisms access intravascular devices: a) contamination of the infusion product; b) contamination of the connection and intraluminal space, and c) contamination of the skin adjacent to the insertion site and the extraluminal surface.
While we know that there are currently no technological or infrastructural advances working on improving central venous catheters or prevention measures in CVAD-related bloodstream infections, we understand the importance of this issue due to the high incidence being observed worldwide. At DIPITS, we are working to promote and educate in this area because as nursing students, we know firsthand what it means to have these pathologies present and the importance of a device focused on this issue being available to the public to improve the quality of life of individuals not only in the hospital setting but also in clinics and homes, allowing for a significant improvement in their quality of life.
DIPITS products offer a promising solution to this problem. By using user-friendly technology that can be accessed by anyone trained or healthcare personnel, individuals can acquire the necessary knowledge and skills to act, care for, and prevent bloodstream infections, as these are a significant issue. DIPITS represents an important step in promoting public health and safety, one that is long overdue.
Background
A study conducted in 2017 by García-Muro et al. in Spain found that only 25% of surveyed nursing professionals had adequate knowledge about the prevention of central venous catheter (CVC)-related infections, and only 15% were familiar with clinical guideline recommendations. Furthermore, it was found that only 12% of participants had received specific training on CVC insertion and maintenance (García-Muro et al., 2017).
In another study conducted in the United States in 2018 by Smith et al., it was found that the rate of bloodstream infections associated with CVCs varied significantly among different Intensive Care Units (ICUs), with an incidence rate ranging from 1.2 to 3.7 infections per 1,000 catheter-days. Additionally, it was discovered that the use of sterile barrier devices during CVC insertion was associated with a significant reduction in infection rates (Smith et al., 2018).
In a study conducted in Canada in 2019 by Patel et al., it was found that the implementation of a CVC-associated bloodstream infection prevention protocol led to a 50% reduction in the incidence rate of these infections over a six-month period. The protocol included staff education on CVC insertion and maintenance practices, as well as the use of sterile barrier devices and regular compliance checks (Patel et al., 2019).
In a study conducted in Australia in 2020 by Lee et al., it was found that the use of chlorhexidine-alcohol dressings during CVC insertion was associated with a significant reduction in bloodstream infection incidence. Compared to chlorhexidine-gluconate dressings, chlorhexidine-alcohol dressings showed a 60% reduction in infection rates (Lee et al., 2020).
Importance of Preventing CVC-Associated Bloodstream Infections
CVC-associated bloodstream infections are a serious complication that can result in significant morbidity and mortality among hospitalized patients. According to data from the Centers for Disease Control and Prevention (CDC), an estimated 250,000 cases of CVC-associated bacteremia occur in US hospitals each year, resulting in over 30,000 deaths (CDC, 2021).
It is important to note that these infections can largely be prevented through the implementation of evidence-based CVC insertion and maintenance practices, as well as the use of appropriate sterile barrier devices and antiseptics. The results of several studies have shown that staff education, compliance with prevention protocols, and the use of specific technologies and products can significantly reduce the incidence of CVC-associated bloodstream infections, thus improving patient safety and quality of care.
In addition to standard CVC care, the implementation of innovative technologies can complement infection prevention efforts. With DIPITS, we show that our device is useful as it is an ultraviolet (UV) light photodisinfection system specifically designed to decontaminate the CVC insertion site. This device uses UV-C light to eliminate pathogenic microorganisms present on the skin and around the catheter, thus reducing the risk of CVC-associated infections. UV photodisinfection offers a quick, effective, and non-invasive way to decontaminate the CVC insertion site, complementing standard care and improving patient safety.
It is very important to address this issue as the morbidity and mortality figures worldwide are alarming. Worldwide, it is estimated that healthcare-associated infections, including those related to CVCs, contribute significantly to hospital morbidity and mortality. According to the World Health Organization (WHO), it is estimated that each year at least 2 million people worldwide acquire healthcare-associated infections, resulting in approximately 90,000 annual deaths in Europe and up to 99,000 annual deaths in the United States. Many of these infections are considered to be associated with medical devices, although not all cases are directly related to CVCs specifically (World Health Organization, 2019).
In terms of morbidity, CVC-associated bloodstream infections can prolong hospital stays, increase healthcare costs, and lead to additional complications for patients. The severity of these infections can vary depending on various factors, including the type of microorganism involved, the overall health of the patient, and how promptly the infection is detected and treated.
It is important to note that the morbidity and mortality figures specifically attributable to CVC-associated bloodstream infections can vary significantly depending on the geographic region, local healthcare practices, and other contextual factors. Local and regional epidemiological studies often provide more accurate data on the incidence and impact of these infections in specific populations.
Changing the Perception:
It is crucial to change people’s perception that getting a central venous catheter is synonymous with death. This is something that DIPITS has been working on, promoting continuous information and education on bloodstream infections on social media, being the first outreach network with the population, as well as with international institutions. By implementing technology that facilitates such learning so that knowledge can be acquired in a more didactic and simple way for everyone.
As mentioned earlier, the actions proposed by the WHO and other companies and organizations for the maintenance of central venous catheters and the prevention of bloodstream infections are merely action measures, which include:
- – Educating healthcare personnel about the indications for CVC use, proper procedures for insertion and maintenance, and appropriate infection control measures to prevent BSIs.
- Using checklists.
- Periodically assessing the knowledge and adherence to guidelines for all personnel involved in CVC insertion and maintenance.
- Designating only qualified personnel demonstrating competence for CVC insertion and maintenance.
- Employing aseptic technique for catheter insertion, which includes surgical hand hygiene and the use of maximal sterile barriers such as cap, mask, sterile gown, goggles, sterile gloves, and full-body sterile drape.
- Performing skin disinfection preferably with 2-4% chlorhexidine (alternative option is 70% alcohol, not iodopovidone for prolonged action initiation).
- CVCs inserted with non-aseptic technique should be replaced as soon as possible, before 48 hours.
- Using new sterile gloves before using the new CVC when exchanging under wire guidance.
- Removing unnecessary CVCs.
- Not routinely replacing CVCs; do so only when considered infected.
- Recommending the use of an exclusive lumen for parenteral nutrition.
- Not routinely using anticoagulants to reduce infection risk.
- Cleaning the connector with 70% alcohol each time solutions are administered.
- With the above in mind, we ask that you not fear change and help us improve the quality of millions of people worldwide, as well as prevent countless deaths. Thank you for your attention.

References:
– World Health Organization (WHO)
– Centers for Disease Control and Prevention (CDC)
– Garcia-Muro et al. (2017). Knowledge of nursing professionals about the prevention of infections related to central venous catheters.
– Smith et al. (2018). Variability in Central Line-Associated Bloodstream Infection Rates Between Different Adult Intensive Care Units.
– Patel et al. (2019). Implementation of a Central Line-Associated Bloodstream Infection Prevention Bundle in Canadian Intensive Care Units.
– Lee et al. (2020). Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis.
Farina, J., Cornistein, W., Balasini, C., Chuluyan, J., & Blanco, M. (2019). Infecciones asociadas a catéteres venosos centrales: Actualización y recomendaciones intersociedades. Medicina, 79(1), 53–60. http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S0025-76802019000100008
Ferrer, C., & Almirante, B. (2014). Infecciones relacionadas con el uso de los catéteres vasculares. Enfermedades infecciosas y microbiologia clinica, 32(2), 115–124. https://doi.org/10.1016/j.eimc.2013.12.002
(S/f). Com.mx. Recuperado el 12 de mayo de 2024, de http://himfg.com.mx/descargas/documentos/planeacion/Guias/GtrataBACTEREMIA_AS_VENOSOS_CENTRALES.pdf

